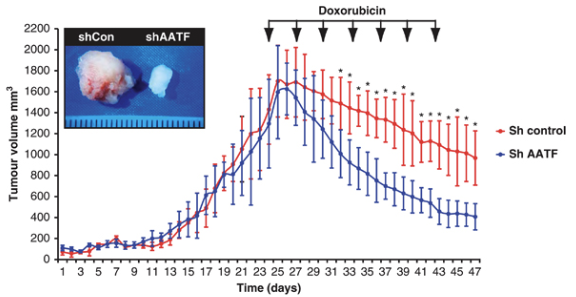
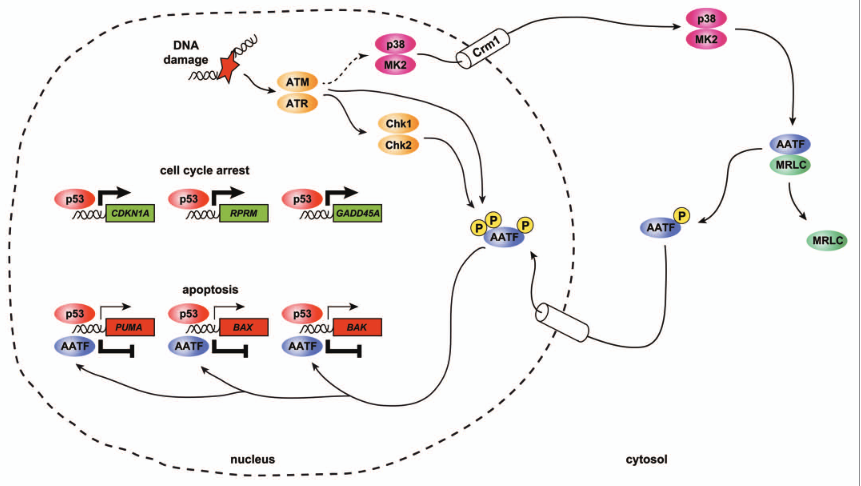
Khurshid Lab
Safiya Khurshid is a trained cancer biologist dedicated to advancing the understanding of tumor biology to drive drug development and innovative cancer therapies. Her research journey began at the University of Hyderabad, where she worked on the biology of malarial parasites under the guidance of Dr. Arun Kumar Kota. This formative experience ignited her passion for scientific discovery and propelled her toward pursuing a PhD at CECAD, University of Cologne in Germany.
Under the mentorship of Drs. Katja Hoepker and Thomas Benzing, Safiya delved into the intricacies of DNA damage signaling and cancer biology, honing her expertise in foundational cancer research. She then pursued postdoctoral training with Dr. Gustavo Leone, a prominent leader in cancer biology. During this time, Safiya expanded her horizons into RNA biology and transitioned to working with Dr. Dawn Chandler. Under the mentorship of both postdoctoral advisors, Safiya not only developed her ability to conduct rigorous and impactful science but also gained invaluable insights into the art and discipline of scientific inquiry, critical analysis, grant writing, and lab management.
Safiya’s expertise spans cancer genetics, tumor microenvironment, RNA biology, and alternative splicing, equipping her with a versatile technical skill set and a profound understanding of cancer's complexities. Her lab leverages this knowledge to unravel the intricacies of tumor biology and develop novel therapeutic strategies, aiming to make a transformative impact in the fight against cancer.

About Khurshid Lab
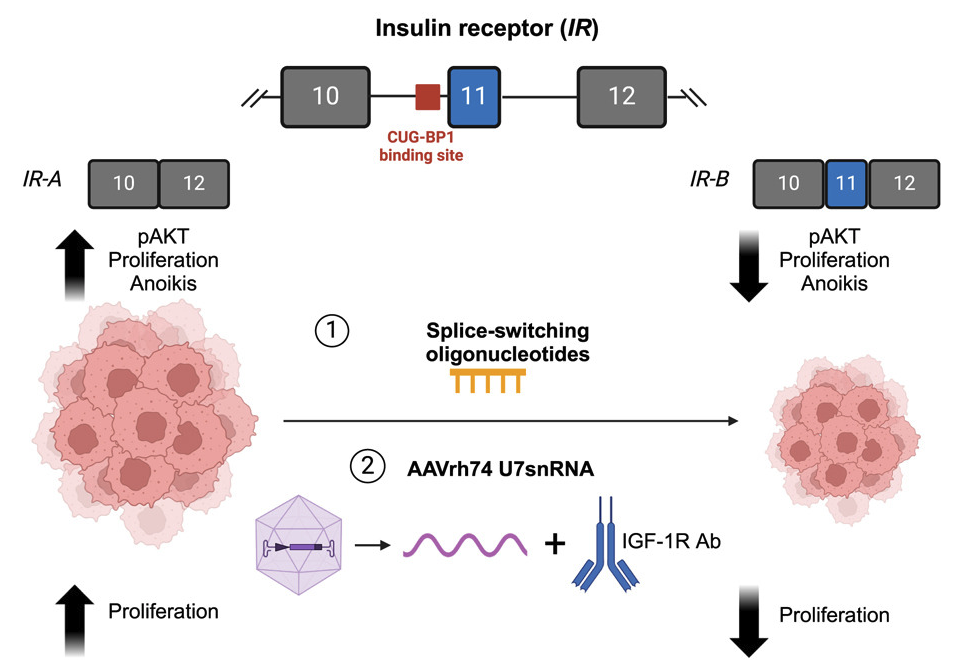
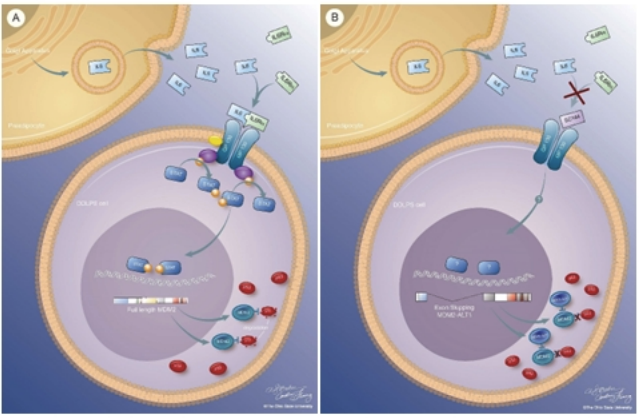
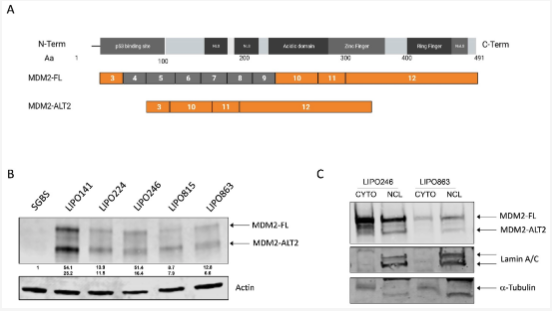
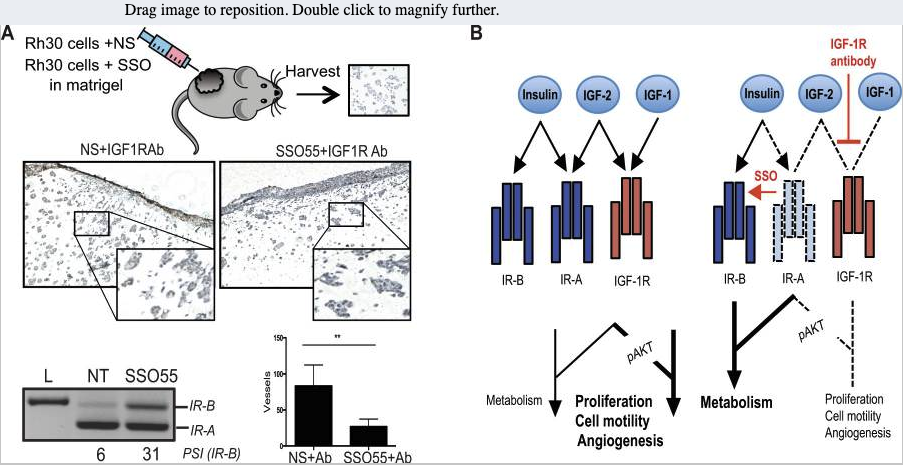
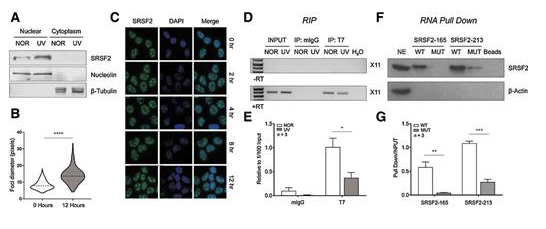
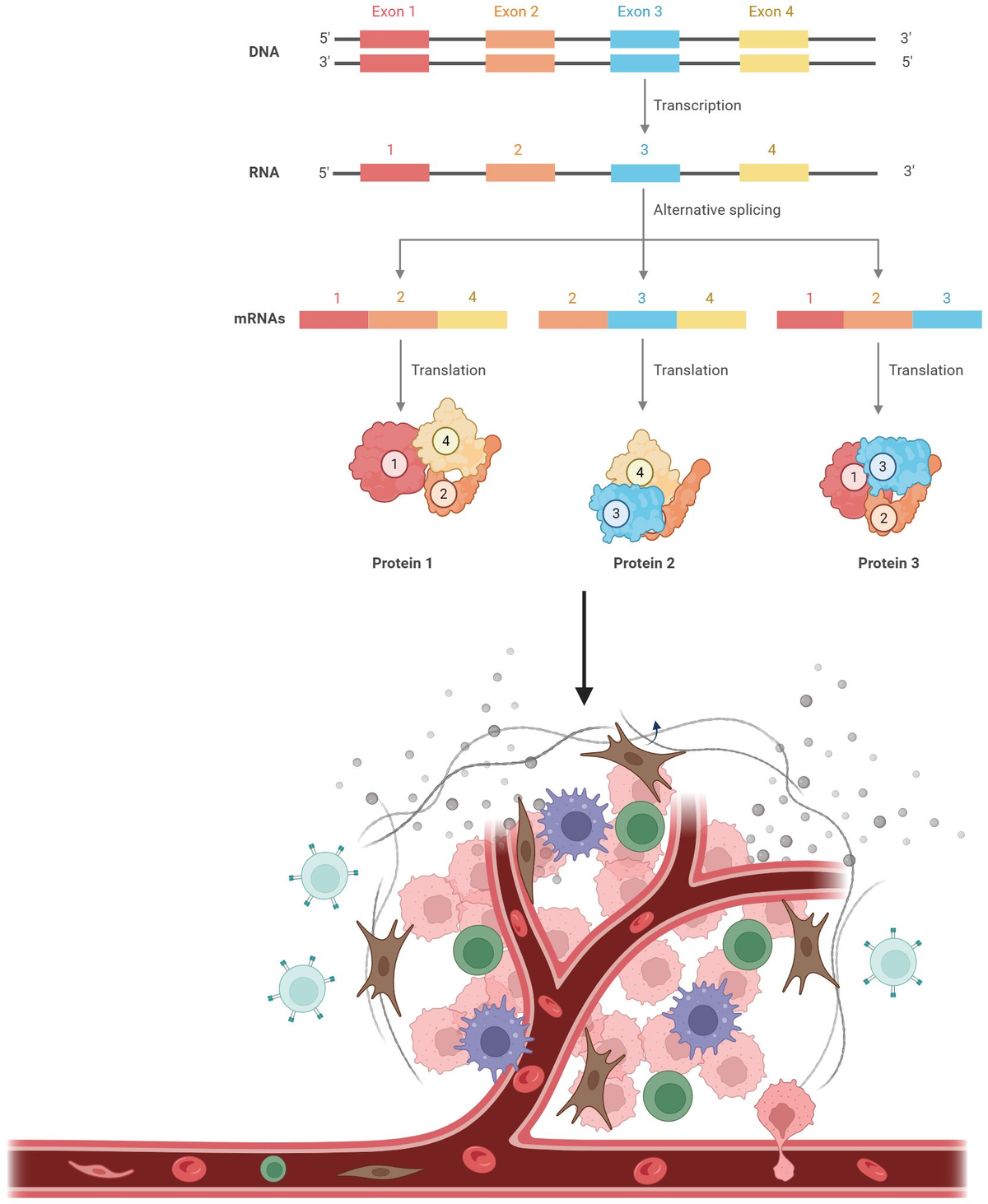
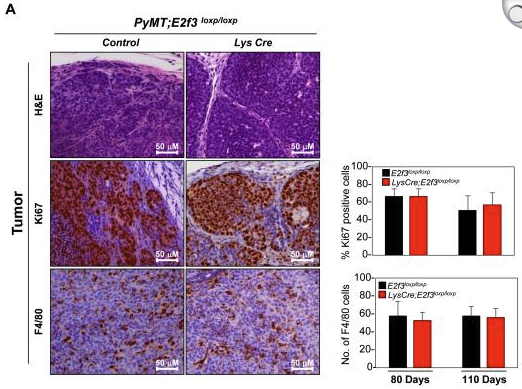
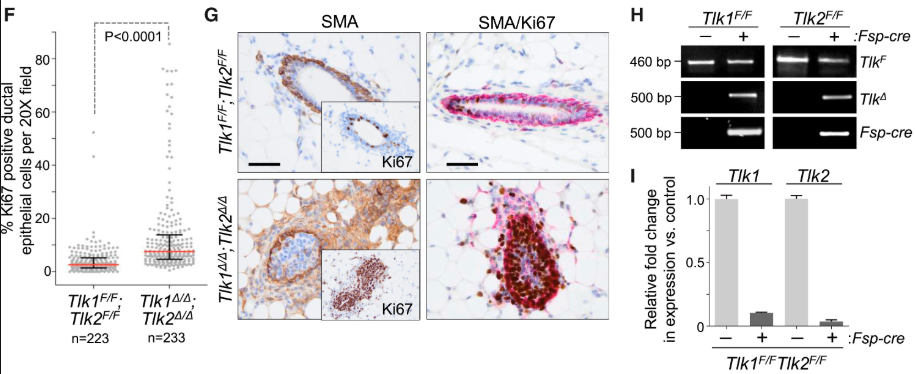
Alternative splicing is frequently dysregulated in cancer, contributing to tumor progression and therapy resistance. Our goal is to identify the isoforms that are differentially expressed in tumors and elucidate the RNA-binding proteins responsible for mediating these changes. Using long-read sequencing technology, we aim to precisely characterize these isoforms in stressed cells and patient samples, paving the way for targeted therapies and adding a new dimension to cancer treatment strategies.
We believe in optimism, relentless hard work, and unwavering focus. We are committed to making meaningful progress and driving innovation to reshape the future of cancer research and eventually improve patient care. Equally vital to our mission is educating the next generation of scientists - fostering curiosity and equipping them with the tools to solve the challenges of tomorrow.
Our Philosophy
At the Khurshid Lab, we embrace a growth mindset, one that values curiosity, resilience, and initiative. We believe that meaningful science blends substance with creativity and is strengthened when we lead with kindness and collaboration. Our philosophy is inspired by an elementary school book that teaches the idea of having a “bubble gum brain”: a flexible, adaptable mind that can stretch, adjust, rethink, and innovate, rather than remaining fixed and rigid. This spirit guides our research, our teamwork, and the way we mentor the next generation of scientists.